Your cart is currently empty!

Understanding the Reaction: HCOOH + CH₂ → H₂O
I
Chemical reactions constitute a large part of industrial and laboratory work. A very interesting reaction between formic acid (HCOOH) and methane (CH₂) gives rise to water (H₂O). This is important in the field of organic chemistry, energy production, and environmental science. In this post, the reaction mechanism, its applications, and its importance to various industries will be discussed.
Understanding The Reactants
Formic Acid: HCOOH
Formic acid is the simplest carboxylic acid and is represented by the chemical formula HCOOH. It is produced naturally from the poison of ants and is largely used in today’s industry. It has a strong pungent smell and is a colorless liquid at room temperature. Formic acid is a strong acid, so it is very reactive in its chemical behavior and is involved in several reactions.
Methane: CH₂ or CH₄?
Methane is the simplest hydrocarbon and is expressed with the chemical recursion CH₄. This should mean that methane is just one carbon atom with four bonded hydrogen atoms. However, from the form of the reactant represented, CH₂ certainly does not exist on its own and might cause some concern. Methane is basically the chief component of natural gas and is widely used as fuel. Methane is one of the greenhouse gas classes that poses a great deal of harm to the environment.
Breaking Down the Reaction
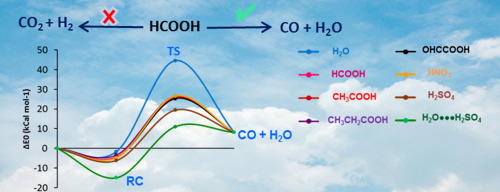
The proposed reaction:
HCOOH + CH₂ → H₂O
points to some reaction between formic acid and methane. Methane (CH₂) is traditionally an incomplete representation of a hydrocarbon in classical chemistry. Methane itself is CH₄, which is its most stable molecular form. This leads to further questions as to whether another related compound can still be reacted.
Possible Interpretations
Decomposition of Formic Acid:
This is possible under certain conditions in which formic acid (HCOOH) decomposes into carbon monoxide and water:
HCOOH → CO + H₂O
As Reducing Agent:
Sometimes formic acid acts as a reducing agent on reactions with hydrocarbons, although methane (CH₂) is not common in reaction with formic acid under normal conditions.
Formation of Formaldehyde:
The other possibility is the partial oxidation of methane to form formaldehyde and water:
HCOOH + CH₄ → HCHO + H₂O
It could imply, henceforth, that the equation HCOOH + CH₂ → H₂O may require elucidation – or need certain reaction conditions such as catalysts, pressure, or temperature variation.
Application of Formic Acid Reactions
- While the actual mechanism of reaction still needs further assurances, formic acid plays an important role in various applications:
- Fuel Cells: It is a hydrogen source for direct Formic Acid Fuel Cells (DFAFCs), an emerging clean energy technology.
- Preservatives & Antibacterial: In varieties of food preservation and as antimicrobial agents due to its acidic nature.
- Chemical Industry: It’s an essential mediator for the synthesis of various organic compounds like dyes, pharmaceuticals, and pesticides.
- Non-Polluting: These reactions of formic acid assist in carbon dioxide reduction and thus render a basis for greener chemical processes.
- Methane Activation in Chemical Processes: Allows catalytic reactions forming valuable chemicals where methane can react with a controlled formic acid dosage.
- The Conditions under Which the Reaction Runs
- For methane to react with formic acid, certain reaction conditions must be satisfied:
- Catalysts: Selective oxidation of methane can be enable rs, using relatively benign metal-based catalysts like palladium or platinum.
- High Temp and Pressure: Man-o’-war, methane, generally need elevated reaction temperature (>200°C) and pressure in the facilitation of bond cleavage.
- Oxidizing Agents: Providing often any oxidizmg) agent, for example, O2 or H2O2, can maneuver forward reaction.
Industrial relevance
Industries at present are constantly finding out ways to use methane more effectively since it is an important part of natural gas. The conversion of methane to useful compounds like methanol or formaldehyde is a major point of study. Should formic acid be effectively used in the conversion of methane, it would provide an alternative utilization route for this abundantly available phenomenallyfuels in a way that ensures minimal carbon emissions.
Environmental impact
Given that methane is a potent greenhouse gas, finding an effective process for its conversion into less harmful substances is of utmost importance. Formic acid-mediated reactions, if optimized, could become an environmentally friendly approach to methane utilization. This trend coincides with the global attempts to reduce emissions of methane and to develop sustainable chemical processes.
Conclusion
The same reaction HCOOH + CH₂ → H₂O need to be further scrutinized for feasibility and possible conditions. Be that as it may, there is no denying the importance of formic acid chemistry. From industrial application and sustainable energy solution, formic acid continues to be an invaluable compound toward scientific advancement. Future research may reveal more efficient reaction routes whereby the unique properties of formic acid might take full advantage when combined with hydrocarbons.
Understanding and optimization of such reactions could lead to advancements in clean energy, industrial chemistry, and environmental sustainability. Would you like to explore besides these several reactions of formic acid? Please share your thoughts in the comments!

Leave a Reply